In today’s fast-evolving pharmacovigilance (PV) landscape, managing diverse sources of safety data and ensuring timely, accurate intake is more critical—and more complex—than ever. That’s where UltraIntake steps in: Ultragenic’s comprehensive case intake solution designed to unify, streamline, and automate the intake process across a wide range of channels.
Whether you’re handling structured web forms, scanned PDFs, or social media inputs, UltraIntake offers an integrated platform to simplify case intake and triage, while enhancing visibility, compliance, and control.
What Is UltraIntake?
UltraIntake is Ultragenic’s AI-powered Case Intake product, purpose-built to manage incoming Individual Case Safety Reports (ICSRs) and safety-related information from multiple sources—intelligently, efficiently, and at scale.
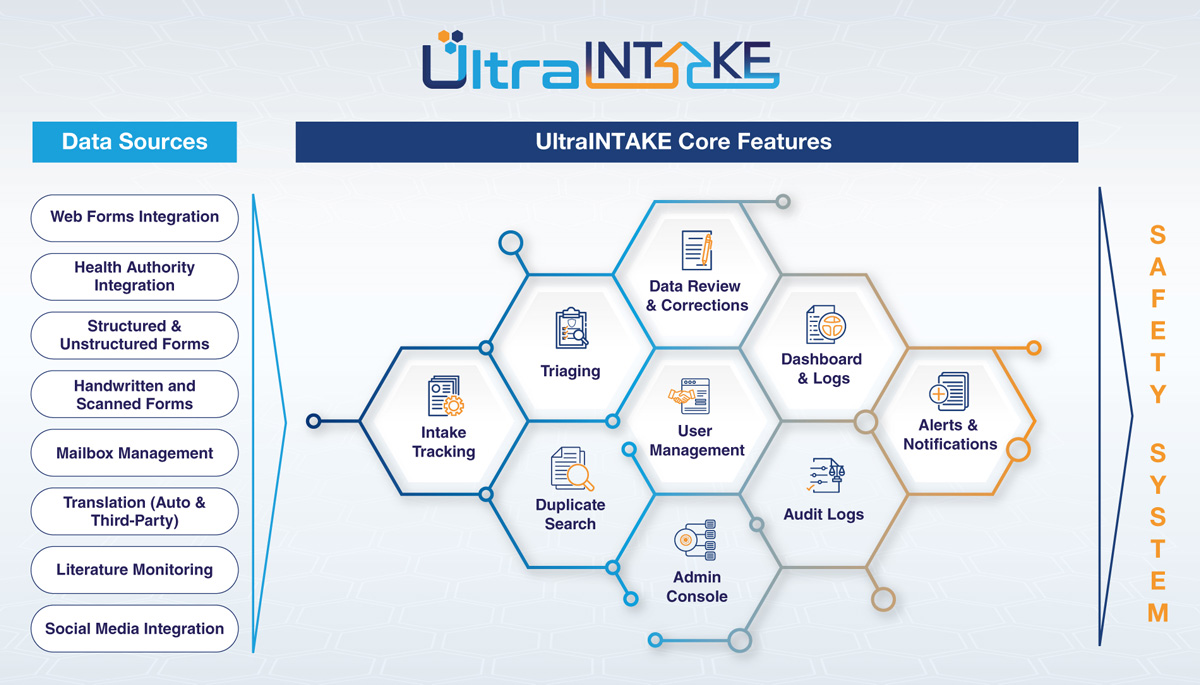
Key Intake Modules
UltraIntake brings together the full spectrum of intake sources, including:
- Web Forms Integration – Seamless intake from online submissions / Customizable, Secure, Multilingual Web Forms Integration
- Health Authority Integration – Direct data flow from regulatory bodies
- Structured & Unstructured Forms – Supports both standardized and free-text formats
- Handwritten and Scanned Forms – Intelligent processing using OCR and NLP
- Mailbox Management – Centralized email intake and routing
- Translation (Auto & Third-Party) – For global case intake
- Literature Monitoring – Integrated surveillance of scientific publications
- Social Media Integration – Captures potential safety signals from public platforms
Core Features That Drive Efficiency
UltraIntake is not just a data collector—it’s a full-service intake engine. Here’s how it enhances your workflow:
- Unified dashboard for all incoming cases
- Real-time case update refresh
- Clear visibility on case status and failure reasons
- Source-to-safety system case number mapping
- Automated case prioritization
- Accept or reject cases based on set rules
- Export listings and assign to users or teams
- Smart algorithms to detect potential duplicates
- Compare duplicates side-by-side with incoming data
- Option to flag as follow-up
- Fully configurable to client needs
- Built-in MedDRA and product coding
- Full data entry view for error corrections
- Auditable correction workflows and reports
- Role-based access with support for LDAP and SSO
- Configurable restrictions by data source, country, or content
- Easy setup of new sources, forms, and sessions
- System-level configurations (LDAP, SMTP, SFTP)
- Real-time performance dashboards
- Source-wise case analysis
- Non-compliance reports and executive-level insights
- Complete audit trail for all data changes
- Generate case-level audit reports
- Compare audit versions with reasoning
- Real-time push and email alerts
- Failure alerts, due case notifications, and assignment alerts
Why Choose UltraIntake?
With UltraIntake, PV teams gain a unified, intelligent platform that ensures nothing falls through the cracks—improving compliance, reducing manual overhead, and speeding up decision-making.
Whether you’re scaling globally, integrating new data sources, or navigating regulatory pressures, UltraIntake empowers you to manage it all efficiently.
Ready to reimagine your case intake process?
Reach out to us at contactus@ultragenicglobal.com to learn how UltraIntake can support your pharmacovigilance operations.